Mozobil is a mobilization agent for those who have been diagnosed with non-Hodgkin’s lymphoma (NHL) or multiple myeloma (MM) and are expected to eventually undergo an autologous hematopoietic cell transplantation (autologous HCT).
As a person’s own cells will be used in autologous HCT, a transplant team must collect them prior to treatment for cancer and keep them until transplant. Mozobil helps to release stem cells from the bone marrow into the bloodstream so they can be collected for transplant through a process called stem cell mobilization.
In clinical studies, Mozobil + [growth factors] demonstrated several benefits for the mobilization of stem cells compared to filgrastim alone.
Mozobil + filgrastim was found to significantly reduce mobilization failures vs filgrastim alone in two well-controlled trials evaluating safety and efficacy
Percentage of patients with MM who achieved the target stem cell collection goal* in 2 or fewer days of apheresis with Mozobil + Filgrastim(n=148) vs placebo + filgrastim (n=154), respectively
Percentage of patients with NHL who achieved the target stem cell collection goal‡ in 4 or fewer days of apheresis with Mozobil + Filgrastim (n=150) vs placebo + filgrastim (n=148), respectively
*Target=6 million CD34+ cells/kg
‡Target=5 million CD34+ cells/kg
Significantly higher success rates for mobilizing stem cells was seen with Mozobil + Filgrastim compared to placebo + filgrastim
Potential for fewer apheresis procedures
Talk to a doctor or healthcare provider about whether or not Mozobil should be taken while:
People who have leukemia should not receive Mozobil. Taking Mozobil to increase stem cells while you have leukemia may increase the number of leukemic cells in the bloodstream.
Mozobil can harm the unborn child when administered to a pregnant woman. If you are a female or male of reproductive potential you should use an effective form of contraception during treatment with Mozobil and for one week following cessation of treatment. Breastfeeding is not recommended during treatment with Mozobil and for one week after the final dose.
Stem cell mobilization is a process whereby stem cells are stimulated out of the bone marrow space (eg, the hip bones and the chest bone) into the bloodstream so they are available for collection for future reinfusion. Learn what happens during stem cell mobilization.
Mozobil is to be administered as a subcutaneous injection approximately 11 hours prior to the start of each apheresis session for up to 4 consecutive days.
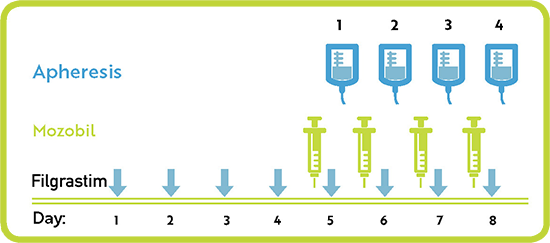
For patients taking Mozobil with filgrastim, the median number of days required to reach the target number of stem cells was 1 for patients with MM and 3 for patients with NHL.
You are most likely to receive Mozobil at your transplant center or hospital depending on hours of operation.
Mozobil is to be used in combination with filgrastim. Filgrastim is administered in the morning daily for 4 days prior to the first dose of Mozobil and on each morning prior to apheresis.