Dosage Forms and Strengths
Mozobil (plerixafor) injection (NDC Number 0024–5862–01) is provided as a 20 mg/mL solution in a single-dose vial. Each vial is filled to deliver a volume of 1.2 mL and contains 24 mg of drug and 5.9 mg sodium chloride dissolved in Water for Injection adjusted to a pH of 6.0–7.5 with hydrochloric acid and with sodium hydroxide, if required.1
Dosing Mozobil
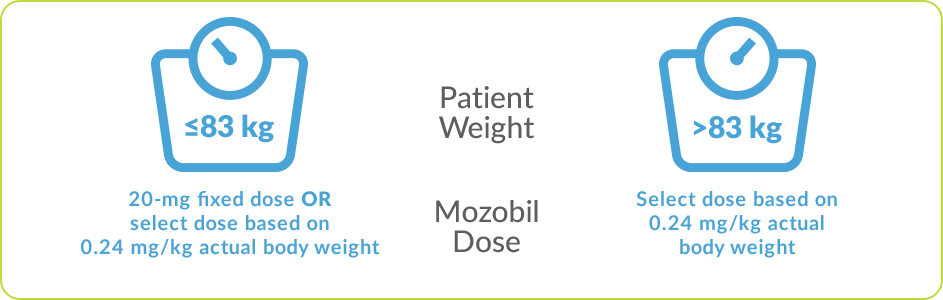
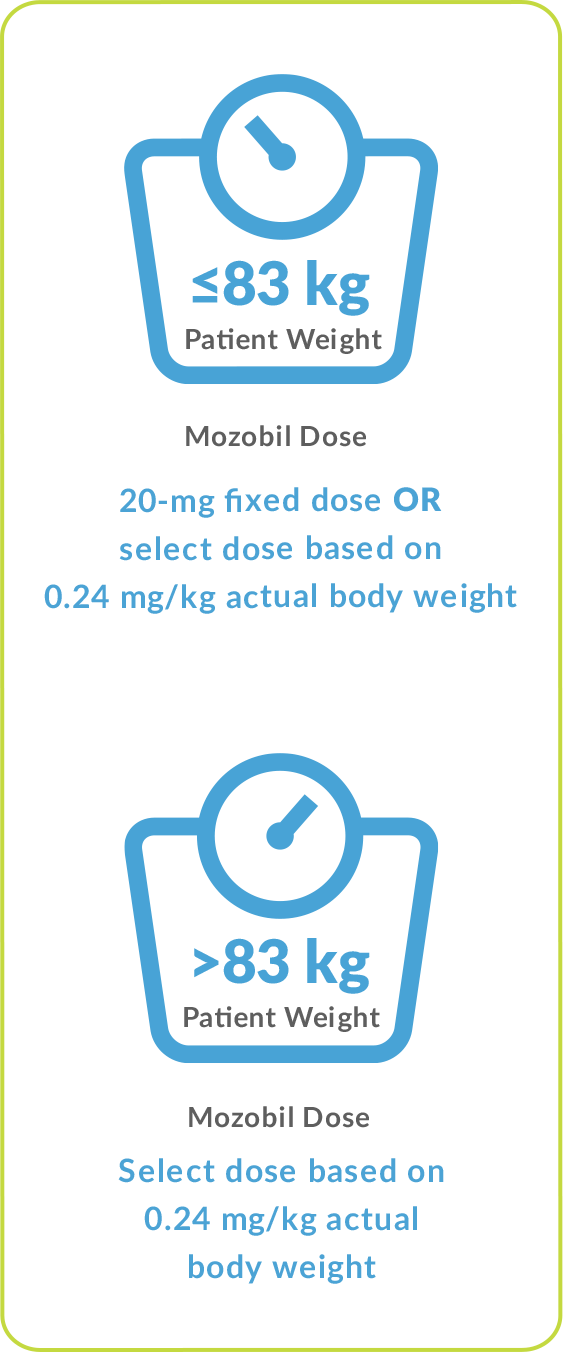
The recommended dose of Mozobil by subcutaneous injection is based on actual body weight, not to exceed 40 mg/day.1
Recommended dose of Mozobil in patients with renal impairment (≤50 mL/min creatinine clearance)1
- Patient weight ≤83 kg: 13-mg fixed dose or 0.16 mg/kg once daily
- Patient weight >83 kg and <160 kg: 0.16 mg/kg once daily (not to exceed 27 mg/day)
Administering Mozobil
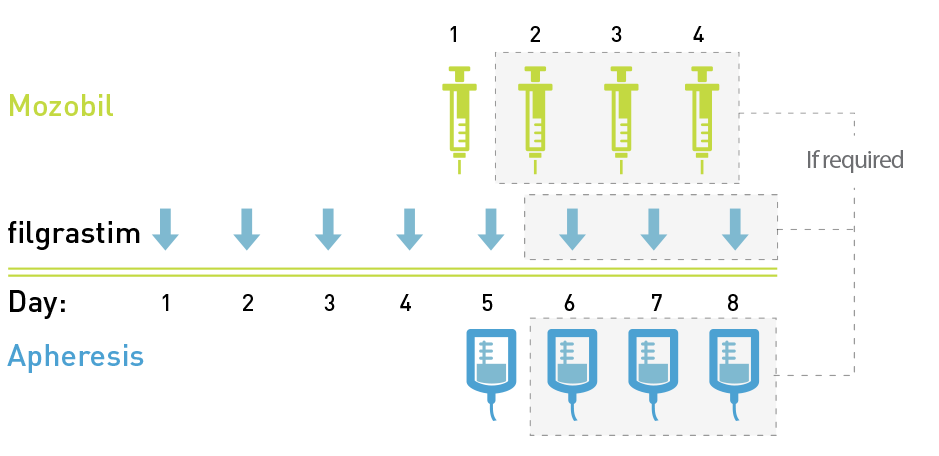
- Begin treatment with Mozobil after the patient has received filgrastim once daily for 4 days1
- Administer Mozobil approximately 11 hours prior to initiation of each apheresis session for up to 4 consecutive days1
- Patients should be monitored for signs of hypersensitivity during and after Mozobil infusion for at least 30 minutes and until clinically stable. Anaphylactic shock and serious hypersensitivity reactions, some of which have been life-threatening, have occurred in patients receiving Mozobil. Observe patients for signs and symptoms of hypersensitivity during and after Mozobil administration for at least 30 minutes and until clinically stable. Only administer Mozobil when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions1
Pharmacodynamic Studies With Mozobil Demonstrating Peak Collection Time of CD34+ Cells
Peripheral CD34+ Cells Collected After Administration of Mozobil + Filgrastim2,*
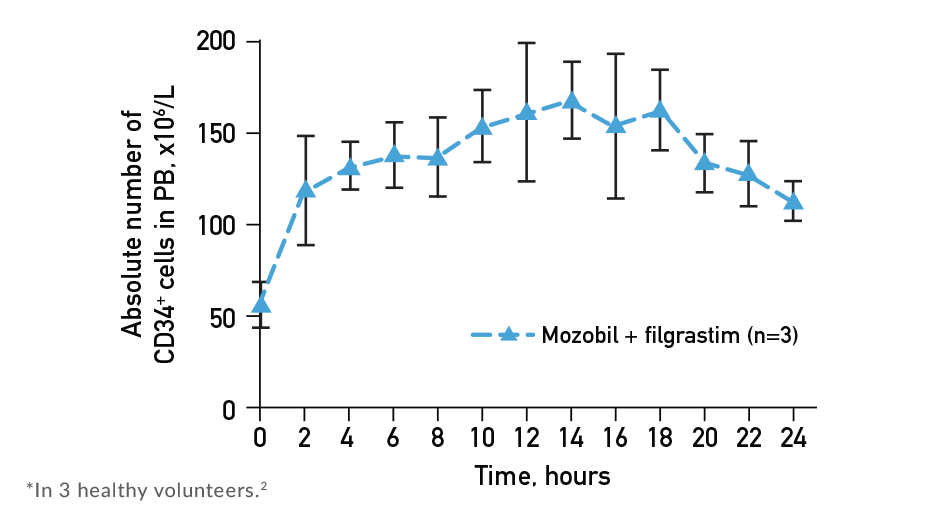
- The 11-hour time point in the Mozobil Prescribing Information was chosen as the peak midpoint1
- Sustained elevation in CD34+ cell count was observed from 4 to 18 hours after administration of Mozobil1,2
- Mobilization and collection of peripheral CD34+ cells after administration of Mozobil + filgrastim peaked between 10 and 14 hours1,2
See the estimated percentage of patients that reached target counts by apheresis day.
Information for Patients Starting Mozobil
Before prescribing Mozobil, advise your patients of the following1:
- The potential exists for anaphylactic reactions, including signs and symptoms such as urticaria, periorbital swelling, dyspnea, or hypoxia during and following Mozobil injection and to report these symptoms immediately to a healthcare professional
- Inform a healthcare professional immediately if symptoms of vasovagal reactions such as orthostatic hypotension or syncope occur during or shortly after their Mozobil injection
- Patients who experience itching, rash, or reaction at the site of the injection should notify a healthcare professional as these symptoms have been treated with over-the-counter medications during clinical trials
- Mozobil may cause gastrointestinal disorders, including diarrhea, nausea, vomiting, flatulence, and abdominal pain. Patients should be told how to manage specific gastrointestinal disorders and to inform their healthcare professional if severe events occur following Mozobil injection
- Female and males of reproductive potential should use effective contraceptive methods during Mozobil use and for one week after cessation of treatment
- Breastfeeding is not recommended during treatment with Mozobil and for one week after the final dose